N-BUTANOL
Used in a wide range of polymers and plastics, as a solvent in a wide variety of chemical and textile processes and as a paint thinner.
Factfile
Name: n-Butanol
Synonyms: butan-1-ol, 1-butanol, butyl alcohol
CAS Number: 71-36-3
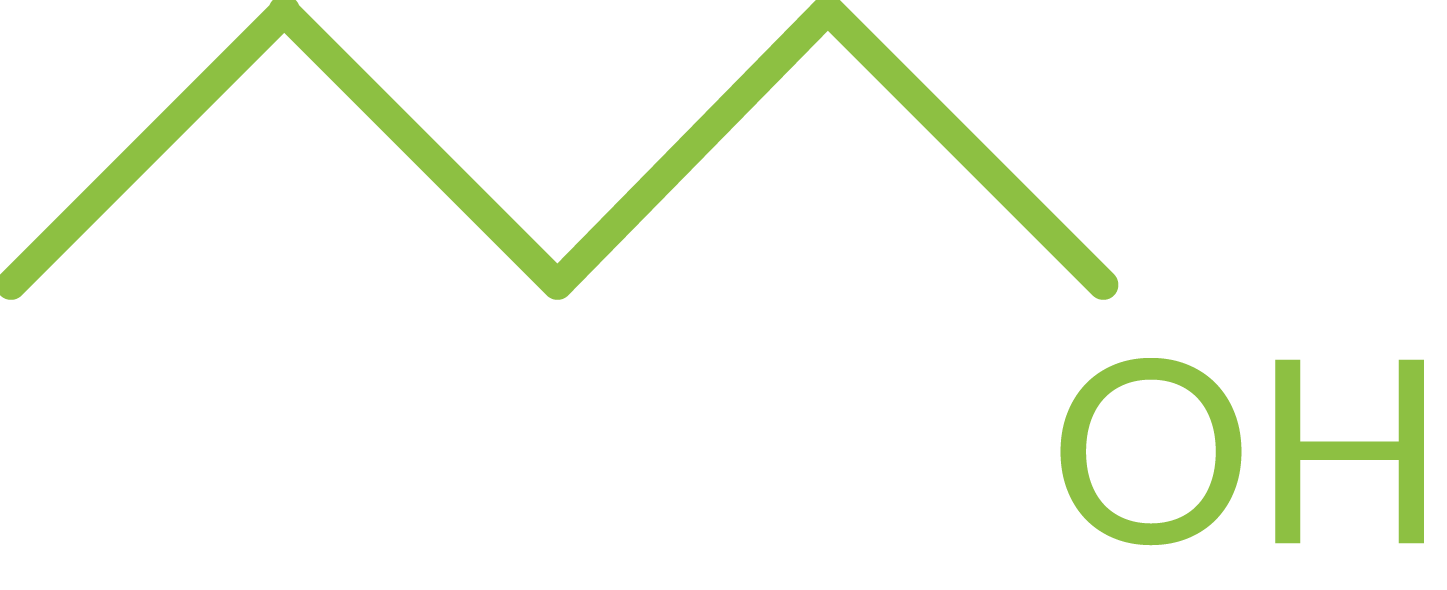
Molecular formula: C4H10O
MW: 74.12 g mol-1
Patents related to synthesis: 279
Why is it of interest?
Butanol is a simple C4 primary alcohol that is a promising bio-fuel for replacement/blending with petrol as opposed to the more commonly used alternative. This is as it has a higher energy content (29.2 MJ dm³), lower volatility (117 °C) and is not as soluble in water compared to ethanol, it also has a comparable octane number (87) to that of petrol (95-98). Butanol is recognised protic solvent, with wide use within the paints and coatings industry. It is also employed as a reactive solvent in the formation of esters, with butyl acetate and butyl acrylate being notable high volume examples. Functional change of the -OH group can yield a variety of useful derivatives, with dehydration to 1-butene a prominent example.
Feedstocks
n-Butanol is already produced on an industrial scale from fossil resources via propylene hydroformylation. However before this process became established in the 1950, fermentation was the principal production route for butanol. This process required bacteria of the clostridium genus and utilised 1st generation feedstock, principally starch. Free sugars can also be used as a feedstock, but require pre-processing, where as corn, sugar cane, sugar beet and potatoes have also all been used to directly produce n-butanol with this bacteria. Second generation biomass feedstock has also been utilised either with pre-treatment followed by hydrolysis to free sugars and then fermentation or by combining the final steps simultaneously. Additionally taking ethanol, dehydrogenating, coupling and then hydrogenating is also an area under much research due to the ease of quantitatively fermenting C2 alcohol from both sugars and increasingly from secondary biomass at industrial scale.
Applications

The major current use of n-butanol is as a solvent, where it is applied in paints and coatings to reduce viscosity and improve film forming. n-Butanol has begun to be incorporated in fuel in the US, but take up has been slower than expected. The reaction of n-butanol with ethylene oxide produces a range of glycol ethers which are widely used in paints, cosmetics and as de-icers by the aviation industry. Other solvents such as butyl acetate also have significant markets. Butanol can be readily esterified to give plasticisers such as dibutyl phthalate (DBP), although this is coming under increased regulation as a suspected endocrine disruptor. Other butyl esters are widely applied, with butyl acrylate and butyl methacrylate being high volume chemicals in the production of emulsion polymers, binders and adhesives.
THE TOP TEN
