MUCONIC ACID
Derivatives could replace non-sustainable chemicals used in the production of PET and nylon fibres.
Factfile
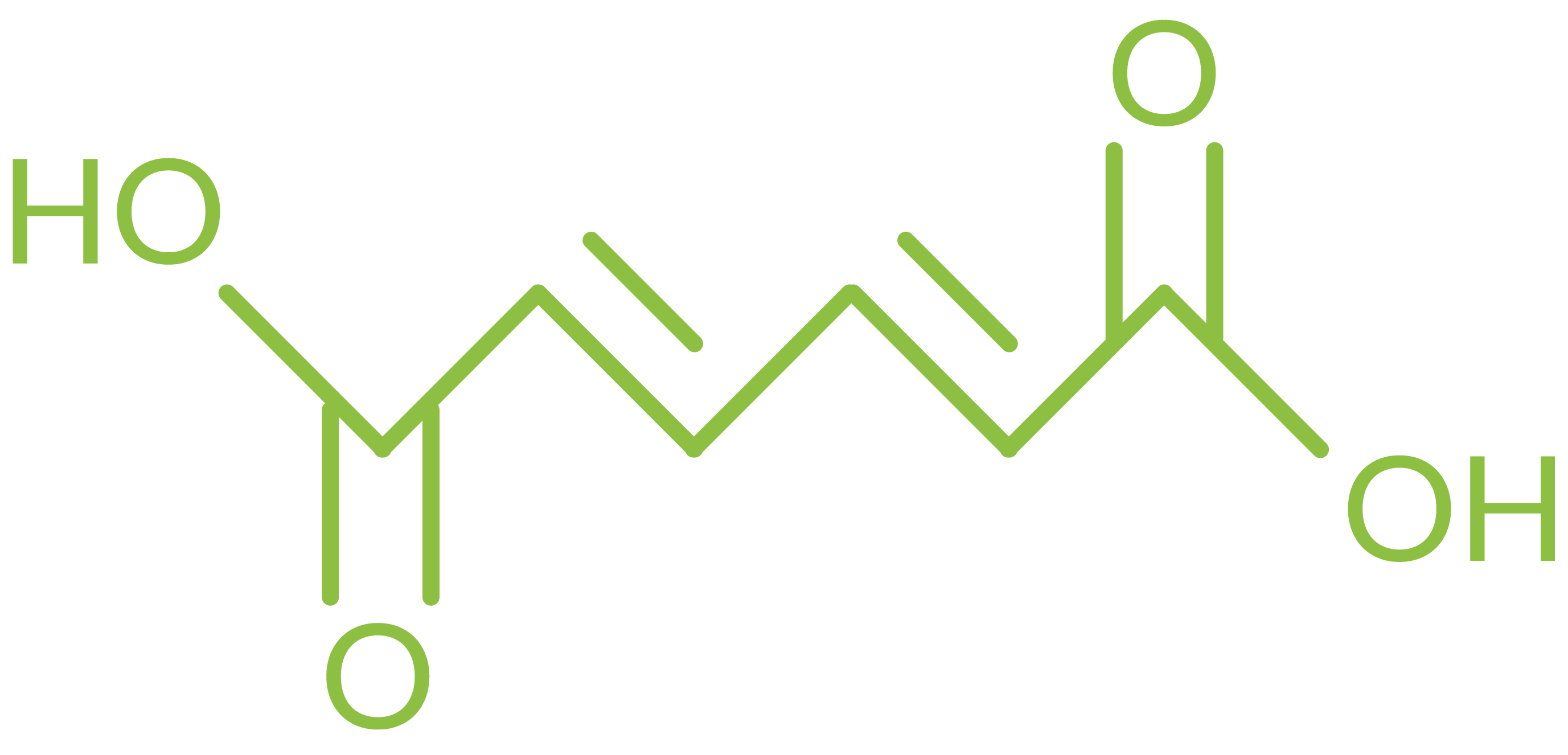
Name: Muconic acid (trans-trans and cis-cis)
Synonyms: 2,4-hexadienedioic acid
CAS Number: 3588-17-8 (trans), 1119-72-8 (cis)
Molecular formula: C6H6O4
MW: 142.11 g mol-1
Patents related to synthesis: 22
Why is it of interest?
Muconic acid (MA) is both a diacid and a diene, giving many options for use as a building-block chemical. The current interest in MA is as a precursor to bio-derived adipic acid, used in the production of nylon 6,6 and other commodity chemicals. MA can be employed as a monomer in the formation of unsaturated polyesters and polyamides in its own right. The double bonds then allow for post polymerisation functionalisation. The dienes also allow for MA to be used in Diels-Alder reactions, most notably as a route to bio-derived terephthalate, used in the production of polyethylene terephthalate (PET).
Feedstocks
Like many other platform molecules, MA can be formed via fermentation of free sugars. Early published work showed a multi step process from glucose using engineered Escherichia coli (E.coli), although here MA was formed as an intermediates, with adipic acid the target compound of the research. The same metabolic pathways were later introduced into other strains of bacteria, but with MA now the desired product. MA has also been produced in good yield from pectin, principally using sugar beet as feedstock. As these pathways all entail the formation of aromatic before ring opening to give the product, other research has focused on utilising aromatic feedstocks in MA production. Initially this focused on model compounds such as benzoic acid, before moving to more complex feedstocks such as coumarates and ferulates, which can be obtained from both softwood and hardwood lignin. This is significant as lignin is available in large quantities, is cheap and is underutilised (predominantly burnt as an energy source) in the production of bio-based products. In fact it would be a by-product of any 2nd generation sugar production facilty such as to give bio-ethanol in addition to numerous suggested fermentation routes to applicable UK BioChem 10 compounds.
Applications

There is no current large-scale usage of MA as it is still relatively novel and early in its exploitation, as stated, all early work focused on a route to adipic acid as this is a substantial commodity chemical, principally used alongside hexamethylenediamine (which can also be obtained from MA) in the production of Nylon 6,6. Additionally MA can be readily converted to caprolactam, of which the major use is polymerisation to Nylon 6, of which 4.6 million tons (petrochemical) was produced in 2016. Another significant commodity plastic is polyethylene terephthalate (PET), the major monomer of which can be obtained via a cascade Diels-Alder reaction between trans-trans-MA (easily obtained via isomerisation), ethanol and ethane. Perhaps of greater future interest is direct co-polymerisation to give a range of unsaturated polyesters which can then be further functionalised, such as cross linking to give thermoset plastics.
THE TOP TEN
