Factfile
Name: Lactic acid
Synonyms: 2-Hydroxypropionic acid, milk acid
CAS Number: 50-21-5 (racemic), 79-33-4 (L), 10326-41-7 (D)
Molecular formula: C3H6O3
MW: 90.08 g mol-1
Patents related to synthesis: 713
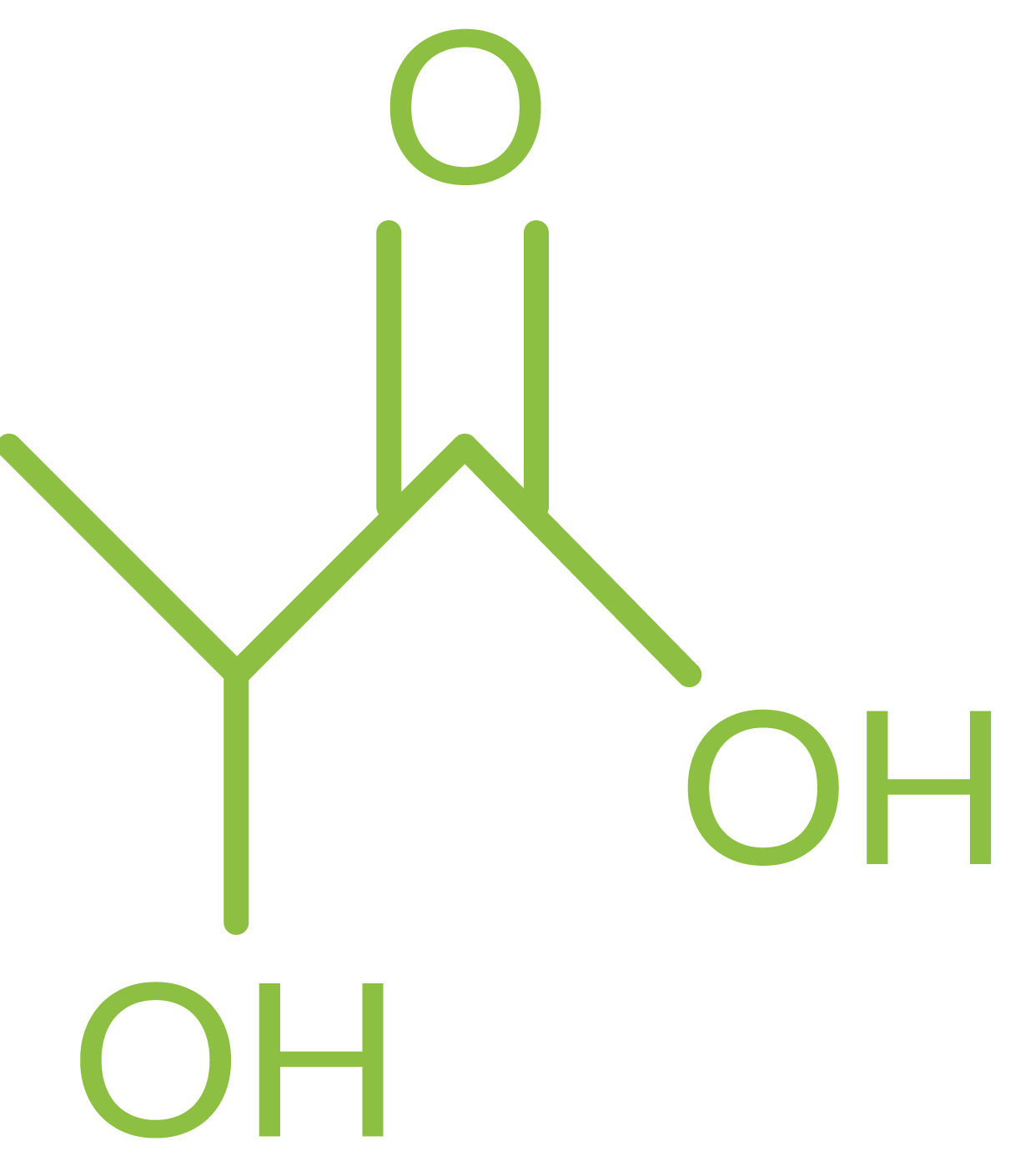
Why is it of interest?
Lactic acid is of interest principally as it contains both an alcohol and a carboxylic acid functional group, both of which can be modified independently. Both funcational groups participate in esterification reactions, meaning it is possible to form diesters, the most significant of these being the self-reaction of two lactic acid molecules to form a cyclic lactide. Through ring-opening polymerisation, the lactide monomer is used to produce polylactic acid (PLA), which is the most important use of lactic acid currently. PLA is a biodegradable plastic with physical characteristics allowing it to be employed in place of some commodity polymers, as well as having medicinal applications. Lactic acid has direct use in the food, pharmaceutical and cosmetics industries as well as being used as a precursor to other bio-derived small molecules.
Feedstocks
Lactic acid can be obtained utilising the petrochemically derived feedstock acetaldehyde, but most commonly comes from bio-derived feedstocks. Originally isolated in the 1780s from sour milk, hence the name milk acid, lactic acid became a commodity chemical in the later 1800s with the discovery of a fermentation pathway. The first industrial fermentation plant was opened in the United States in 1881. Current worldwide production remains dominated by fermentation of carbohydrate sources, principally in the form of free sugars, such as glucose, sucrose, maltose or lactose, or from more complex feedstocks such as starch. This fermentation gives a high purity product to a single target stereoisomer (L-lactic acid as the most common target). Chemo-catalytic routes can accept a much broader range of feedstock, such as rice husks, glycerol (a by-product of bio-diesel production) or sawdust.
Applications

Food applications of lactic acid remain significant, where it is used as a preservative and in the production of emulsifiers used in baked goods. In the cosmetics industry its use is principally to impart firmness or as a filler in anti-aging formulations. The fastest growing and most significant application is in the production of the plastic PLA which is formed by first reacting lactic acid to form a six-membered lactide, followed by ring opening polymerisation. PLA has comparable mechanical, optical, thermal and barrier properties to commodity plastics such as polypropylene, polystyrene and PET. PLA has been applied in extrusion processing, injection moulding, blow moulding, film casting and thermoforming to give a wide array of products. Both the EU and the USA have deemed PLA food safe and Generally Recognized as Safe, which has resulted in increased take up, coupled with its ease of chemical recyclability and biodegradability. Significantly, there are numerous applications in the field of healthcare, where PLA’s biocompatibility and biodegradation, as well as its structural characteristics, ease of production of articles, price and tunability, have made it very attractive. If the PLA is solely produced from the L isomer, it has a high degree of crystallinity, mechanical strength and slow degradation, so has been used as tendon and ligament repair, pins and plates for bone repair, scaffolds for cellular applications and stents. Conversely, PLA produced from a mix of L and D isomers is amorphous and degrades readily so is applied as sutures and in drug delivery systems.
THE TOP TEN
