5 HYDROXYMETHYL FURFURAL (HMF)
A versatile chemical with potential to replace chemicals used in plastics and polyesters, and for producing high energy biofuel.
Factfile
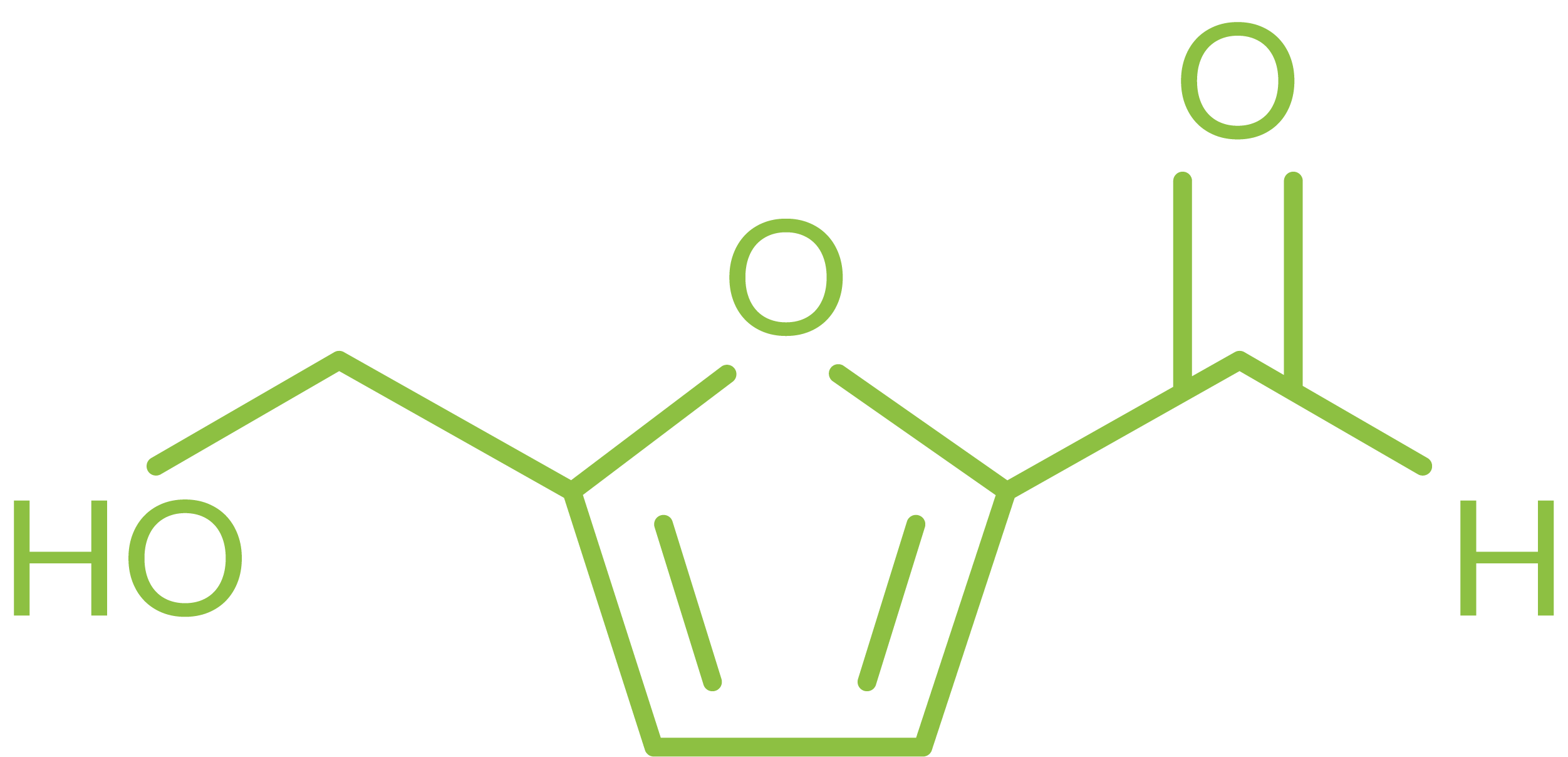
Name: 5-(hydroxymethyl)furfural
Synonyms: 2,5-hydroxymethyl-2-furfuraldehyde, HMF
CAS Number: 67-47-0
Molecular formula: C6H6O3
MW: 126.11 g mol-1
Patents related to synthesis: 134
Why is it of interest?
5-(hydroxymethyl)furfural (HMF) in itself is unlikely to be a commodity chemical, bought and sold on the market as it is too reactive. It is however easily obtained from a range of feedstocks and is a compound containing four key functionalities, a primary alcohol, an aldehyde, unsaturation (two double bonds) and a furan ring. This in turn means there are a diverse number of ways to immediately further react HMF, giving rise to a wide range of important products/intermediates with sufficient stability. In most cases the derivatisation of HMF has been achieved in , or almost in, quantitative yield, i.e. >99% conversion of HMF to the desired product.
Feedstocks
The simplest route to HMF is the dehydration of fructose (fruit sugar) as this is already a 5 membered ring, although this is a high cost, edible feedstock. Fructose can however been also found in polymeric form as the non-digestible oligosaccharide inulin. This is also an excellent raw material for HMF synthesis but is not widely abundant, found in root crops and speciality vegetables such as chicory and artichokes at up to 18% by dry weight. Glucose (6 membered ring) can be isomerised in an acidic environment to give fructose and in turn HMF, as too can its related polysaccharide, starch. Sucrose, more commonly known as table sugar, a disaccharide of glucose and fructose, can also be easily utilised as a route to HMF. The most abundant, cheap and none edible feedstock for HMF however is cellulose. (a polymer of glucose, present in many waste materials).
Applications

2,5-bishydroxymethyl furan has potential in the production of various polymers (foams, fibres, resins) as well as an intermediate in the synthesis of active pharmaceutical ingredients and crown ethers. Further hydrogenation gives 2,5-hydroxymethyl-THF which can be applied as a solvent and has also been used in the production of polyols and as the diol in a range of polyesters. Hexanediol has similar applications. Dimethyl furan has shown potential for use as bio-fuel due to its high energy density (40% greater than that of ethanol), non-miscibility with water and reasonable boiling point. Dimethyl-THF has similar potential for use as a transport fuel, in addition to use as a solvent. Diformylfuran has found application in the production of polymers, adhesives, binders, foams, in furan urea resins, pharmaceuticals, antifungal agents and as ligands in catalyst production. Adipic acid has numerous applications, but the vast majority currently produced is used in the synthesis of nylon 66. Maleic anhydride similarly has a variety of applications, but of most note is in the production of commodity polyesters and as cross-linking (curing) agents in adhesives and resins. Diamines have application in the production of polyamides and polyureas while aminomethyl hydroxylmethyl furans have seen extensive use in the pharmaceutical sector.
THE TOP TEN
