2,5-FURANDICARBOXYLIC ACID (FDCA)
Can be used to make polymers such as PEF, a stronger alternative to PET, which is a fibre used to make plastic bottles, food packaging and carpets.
Factfile
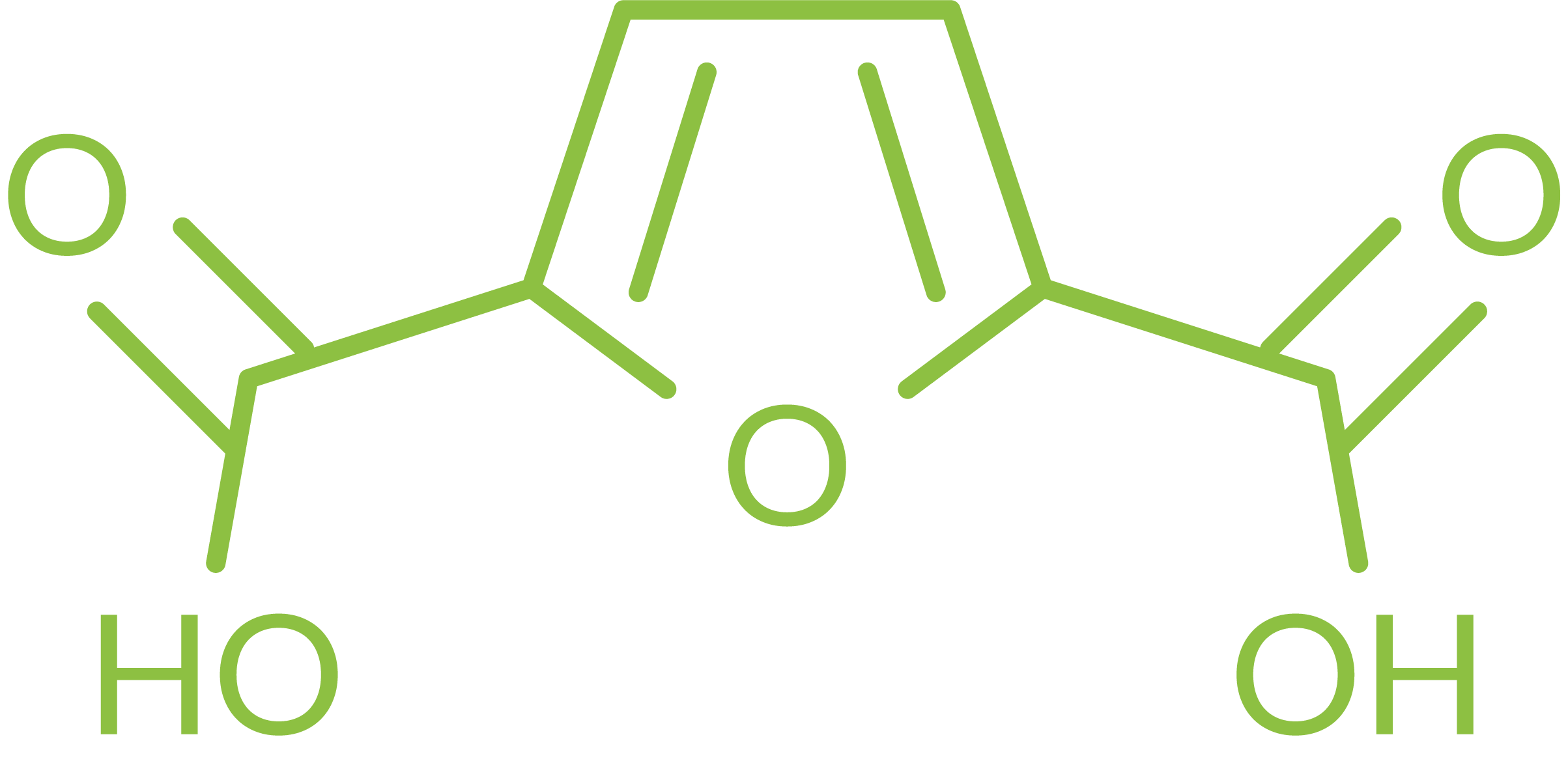
Name: Furan-2,5-dicarboxylic acid
Synonyms: 2,5-dicarboxyfuran, FDCA
CAS Number: 3238-40-2
Molecular formula: C6H4O5
MW: 156.09 g mol-1
Patents related to synthesis: 76
Why is it of interest?
FDCA contains a number of interesting functional groups, the central 5 membered furan structure is an electron rich, planar aromatic ring. This is of great significance as there are very few bio-derived aromatics (the petrochemical industry predominantly utilises 6 member benzene ring aromatic structures). Furans can partake in a wide range of reactions such as cycloadditions and offer routes to other functionality of interest. The second key functionality is the carboxylic acids which can easily give rise to esters, thio-esters, amides, acid halides and acid anhydrides. As FDCA is a diacid, these carboxylic groups can be used in polymer synthesis with diols or diamines to form polyesters and polyamides respectively. It is in this application which FDCA is showing most promise.
Feedstocks
All bio-derived routes to FDCA have the monosaccharide fructose or glucose as their start point. Fructose is the less abundant of the two but is already a 5 membered ring and as such offers an easy route to FDCA via dehydration. Glucose can either directly be converted to FDCA or can easily be converted to fructose. If taken from a primary biomass (i.e. grown for the specific purpose of producing a chemical), both methodologies could encroach on food provision, however such monosaccharides can be obtained from secondary biomass (parts of a crop not used in food production) or unavoidable/inedible food waste to circumvent this. However for ease of analysis, all routes of production are taken from the free sugar.
Applications

FDCA has many potential applications; by far the most interesting is in the production of polyethylfurandicarboxylic acid (PEF) as a bio-derived alternative to polyethyleterephthalate (PET). Early studies of PEF show that it has improved barrier properties than PET, can be applied as a drop in replacement for PET and can be recycled with it at up to 20 weight% with no mechanical detriment. Recently Avantium, along with a consortium of 10 other companies, announced plans in 2017 to build a 50,000 tonnes per annum plant to produce FDCA. Similarly Coca-Cola published the synthesis of PET via the reaction of FDCA with ethane. There are patents on the production of other monomers from FDCA such as diamides and the production of diesters as binders. A number of multinationals have patented the synthesis of more complex diesters, potentially via acid chlorides, for the production of plasticisers. FDCA have been patented in the synthesis of MOFS for the production of novel heterogeneous catalysts in addition to the production of novel ligands for catalytic processes.
THE TOP TEN
